Electrolysis of water
(water => hydrogen and oxygen)
When an electric current is passed through water, water molecules break apart to form its constituent elements, namely hydrogen and oxygen gases.
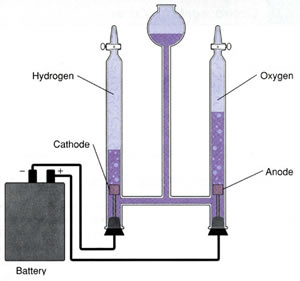
The apparatus used for the electrolysis of water is shown on the right. Hydrogen gas is produced at the negative terminal while oxygen gas is produced at the positive terminal. Click to see the chemical reactions that occur at each electrode.
Solar electricity can be harnessed to break water into hydrogen and oxygen. These gases can later be used in a fuel cell to produce electricity.
View the video on the right.
What is the ratio of the volume of hydrogen produced compared to oxygen?
Explain why this ratio exists with reference to the chemical equation below.
2H2O(l) => 2H2(g) + O2(g)
Notice how each gas is collected.
Why is hydrogen allowed to rise into the collecting test tube?
Why is oxygen allowed to sink into the collecting test tube?
Which of the following is a property of hydrogen gas?
Describe a way that solar energy can be collected and stored on a Martian base and used during night time.
Once the water is electrolysed the hydrogen and oxygen gases can be used as a source of stored energy and used in a hydrogen/oxygen fuel cell to generate electricity.
The video on the right shows the energy conversions from solar to electrical and demonstrates the vital role water plays in the creation of electrical power using a fuel cell.
1) Describe the energy conversions taking place in the device shown on the right. The first and last stages are shown.
Solar --> _____________ --> __________ --> electrical.
2) In what form is chemical energy stored in the device?
3) Some scientists believe that the future energy demands for transport will be provided by hydrogen. Explain how this form of energy, as depicted in the video, is a clean, environmentally friendly energy source.